How Long Does Nausea Etc Continue After Starting 2000iu D3
US Pharm. 2019;44(12):HS10-HS13.
ABSTRACT: Radiation is one of the treatment modalities used for the management of malignancies. One of the side effects of radiation therapy is radiation-induced emesis; however, antiemetics appear to sometimes be underutilized. Several organizations have published guidelines on the management of radiation-induced nausea and vomiting based on the level of risk. Radiation-induced emesis can be well controlled with the use of a 5-hydroxytryptamine type 3 receptor antagonist, reflecting serotonin's role in this condition. Appropriate prevention and treatment of radiation-induced nausea and vomiting are necessary for completing the course of radiation therapy so that a positive treatment outcome may be achieved without interruption.
Radiation-induced nausea and vomiting as a side effect of radiation therapy may be underestimated by both the heathcare provider and the patient.1,2 Radiation can cause nausea and vomiting, which can start during the radiation therapy and last for days after radiation therapy is completed; therefore, it can also affect the efficacy of other cancer treatment modalities, including chemotherapy if it is also a part of the treatment regimen.2
Overview
Nausea is a serious side effect of radiation therapy that can impact the patient's quality of life. It is estimated that radiation induces nausea and vomiting in 50% to 80% of patients. Patients undergoing this therapy often receive up to 40 fractions of radiation in a 6- to 8-week period. Patients with radiation-induced nausea and vomiting potentially could refuse further treatment or experience delays in treatment, which could adversely affect outcome.1
Risk Factors for Radiation-Induced Nausea and Vomiting
The severity of nausea and vomiting induced by radiation therapy depends mainly on the area where the radiation is delivered. Total-body radiation has the highest likelihood of inducing nausea and vomiting, and radiation delivered to the upper abdomen incurs the second-highest risk. Also, increasing the body area being irradiated, the radiation fractions used, and the overall total dose of radiation administered will elevate the risk of radiation-induced nausea and vomiting.2 It has been reported that the use of irradiation sites exceeding 400 cm2 significantly influences the prophylactic use of antiemetic agents to prevent radiation-induced nausea and vomiting.3
Pathophysiology and Mechanisms
The pathophysiology and mechanisms of radiation-induced emesis are complex. It has been suggested that both serotonin levels and the abdomen play important roles in radiation-induced nausea and vomiting. Elevated levels of the active serotonin metabolite 5-hydroxyindoleacetic acid have been identified in the urine of patients experiencing this side effect. Also, emesis caused by radiation therapy can be well controlled with administration of a 5-hydroxytryptamine type 3 (5-HT3) receptor antagonist, which confirms the role of serotonin in radiation-induced nausea and vomiting.4
Antiemetic Prescribing Practices
Statistically significant factors that influence the prescribing of antiemetic therapies are concurrent administration of chemotherapy with radiation therapy and previous occurrence of chemotherapy-induced vomiting. Based on study results, radiation-induced nausea and vomiting appear to be underestimated, and antiemetics for the condition are being underprescribed by radiation oncologists.3
A variety of antiemetic agents have been used for the prevention and treatment of radiation-induced nausea and vomiting, but the most evidence is available for 5-HT3 receptor antagonists.5,6
Guidelines for Management
Three different guidelines on the management of radiation-induced nausea have been developed by the National Comprehensive Cancer Network (NCCN), the American Society of Clinical Oncology (ASCO), and the Multinational Association of Supportive Care in Cancer (MASCC) and European Society for Medical Oncology (ESMO).7-9
NCCN Guidelines: The NCCN lists total-body radiation as having the highest risk of nausea and vomiting because it induces this condition in more than 90% of patients (TABLE 1).7 Of patients who receive upper-abdominal radiation therapy, 30% to 90% will experience nausea and vomiting. Patients undergoing total-body irradiation should receive a 5-HT3 receptor antagonist—either granisetron 2 mg by mouth daily or ondansetron 8 mg by mouth two to three times daily, depending on the patient's needs—with or without dexamethasone 4 mg by mouth daily. In patients receiving radiation to the upper abdomen or other localized sites, either granisetron 2 mg by mouth daily or ondansetron 8 mg by mouth twice daily, with or without dexamethasone 4 mg by mouth daily, should be given.
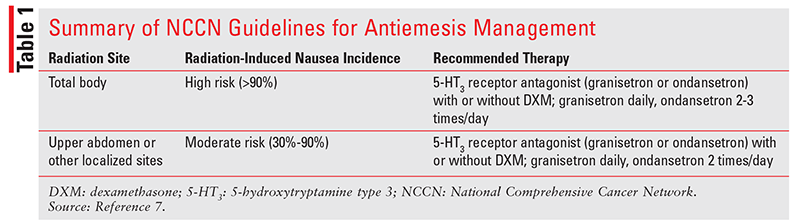
For concurrent administration of chemotherapy and radiation, it is recommended that an antiemetic regimen appropriate for chemotherapy be used. Some chemotherapy agents confer a higher emetogenic risk when they are combined with radiation. For example, temozolomide less than or equal to 75 mg /m2 per day administered orally is considered to have minimal to low emetogenic risk (frequency of emesis <30%), but when it is administered concurrently with radiation, it has a moderate emetogenic risk (frequency of emesis 30% or higher). Patients who experience breakthrough radiation-induced nausea and vomiting should be given a drug of a different class from the agent administered for breakthrough chemotherapy-induced nausea and vomiting.
Patients who experience breakthrough radiation-induced nausea and vomiting should be given a drug of a different class from the agent administered for prophylaxis of the nausea and vomiting.7
ASCO Guidelines: The ASCO guidelines classify radiation-induced nausea and vomiting into four risk categories (high-, moderate-, low-, and minimal-risk) based on the anatomical site of radiation administration (TABLE 2).8 Total-body radiation, which causes nausea and vomiting in more than 90% of patients, is considered high-risk. Craniospinal irradiation and radiation administered to the upper abdomen are moderate-risk, as they induce nausea and vomiting in 30% to 90% of patients. Radiation administered to the brain, head and neck, thorax, or pelvis is considered low-risk because it induces nausea and vomiting in just 10% to 30% of patients. Radiation administered to the extremities or the breast confers minimal risk because it induces nausea and vomiting in fewer than 10% of patients.
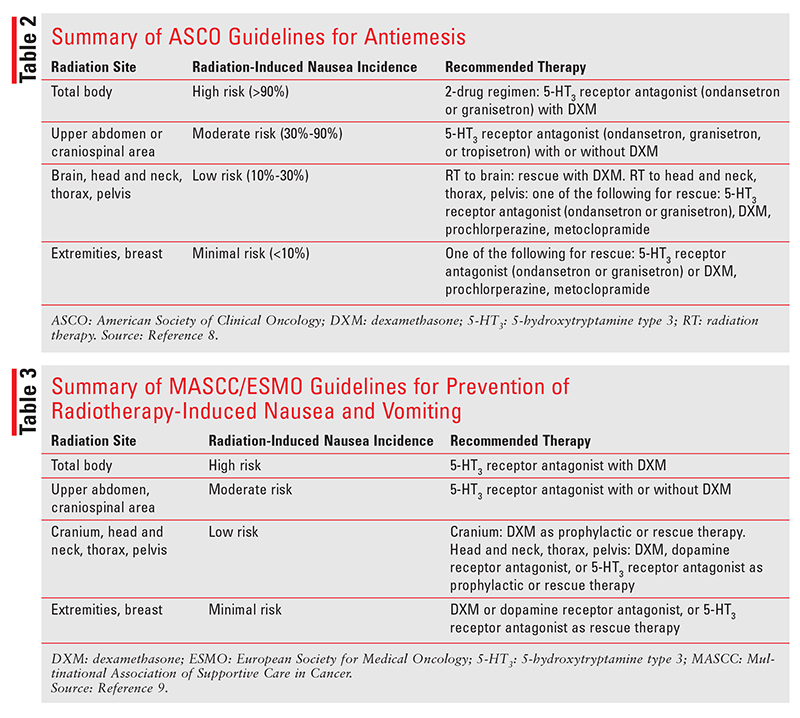
It is recommended that patients who are undergoing high-risk (total-body) radiation be offered a two-drug regimen for prevention of radiation-induced nausea and vomiting. The two-drug regimen should comprise either ondansetron (oral, oral dissolving tablet, oral soluble film, or IV) or granisetron (oral or IV) plus oral or IV dexamethasone. Ondansetron may be administered once or twice daily on radiation-therapy days; if it is administered twice daily, the first dose should be administered prior to the radiation. Also, the patient should receive ondansetron once or twice daily on the day following each radiation session if radiation therapy is not planned for that day. Granisetron is administered once daily on the day of each radiation session prior to administration of radiation and on the day after each radiation session if radiation therapy is not planned for that day. Dexamethasone is administered prophylactically once daily on days of radiation therapy before radiation is administered and on the day following each radiation session if radiation therapy is not planned for that day.
Patients receiving moderate-risk radiation (upper abdominal or craniospinal) should be given prophylactic treatment with ondansetron (oral, oral dissolving tablet, oral soluble film, or IV), granisetron (oral or IV), or tropisetron (oral or IV) and, optionally, oral or IV dexamethasone. Prophylactic ondansetron may be administered once or twice daily on days of radiation therapy, with the first dose administered before the radiation. Granisetron and tropisetron are administered once daily before the radiation. If dexamethasone is used prophylactically, it should be administered once daily prior to the radiation on the first 5 days of radiation therapy.
According to the ASCO, patients undergoing low-risk radiation (head and neck, thorax, or pelvis) or minimal-risk radiation (extremities or breast) should be offered rescue therapy with ondansetron or granisetron, dexamethasone, or a dopamine receptor antagonist (prochlorperazine or metoclopramide). Patients undergoing low-risk radiation to the brain should be offered dexamethasone as rescue therapy. Dexamethasone may be administered as a rescue agent if the patient is not already taking a corticosteroid, and the dexamethasone dose should be titrated as needed to a maximum of 16 mg daily (oral or IV). Prochlorperazine and metoclopramide administered as rescue agents for low-risk radiation-induced nausea and vomiting should be titrated up as needed to a maximum of 3 to 4 administrations daily. For adult patients being treated with radiation therapy and chemotherapy concurrently, the antiemetic regimen should be appropriate for the emetic risk of chemotherapy if the risk level of radiation therapy is not higher than the risk level of chemotherapy. If radiation therapy continues after chemotherapy has been completed, the antiemetic regimen should be adjusted to the emetic risk of radiation therapy.8
MASCC/ESMO Guidelines: The MASCC/ESMO guidelines also classify radiation-induced nausea and vomiting into four categories based on the irradiation site (TABLE 3).9 These categories are similar to those in the ASCO guidelines. The high-risk category comprises total-body radiation; it is recommended that these patients receive prophylactic treatment with 5-HT3 receptor antagonists in combination with dexamethasone. Patients undergoing upper-abdominal or craniospinal radiation therapy are considered to be at moderate risk for developing radiation-induced nausea and vomiting. In these patients, prophylactic treatment with a 5-HT3 receptor antagonist and optional dexamethasone is recommended. Patients receiving radiation to the cranium, head and neck, thorax region, or pelvis are considered to have a low risk of developing radiation-induced nausea and vomiting. Patients receiving cranial radiation should be given dexamethasone as prophylactic or rescue therapy. Those receiving radiation therapy to the head and neck, thorax region, or pelvis should receive prophylactic or rescue therapy with dexamethasone, a dopamine receptor antagonist, or a 5-HT3 receptor antagonist. Patients who receive minimal-risk radiation therapy (extremities or breast) should be offered rescue therapy with dexamethasone, a dopamine receptor antagonist, or a 5-HT3 receptor antagonist. If a patient receives concomitant chemotherapy and radiation therapy, the antiemetic therapy should be aligned with the emetic risk of the chemotherapy unless radiation therapy has a higher risk of inducing nausea and vomiting.9
Conclusion
Radiation-induced nausea and vomiting can be a serious side effect of radiation therapy, adversely impacting the patient's quality of life and potentially compromising treatment because patients who experience severe nausea and vomiting might refuse subsequent radiation therapy treatments. Therefore, prevention and treatment are important to help avoid or alleviate the symptoms of radiation-induced nausea and vomiting. Various resources are available to assist healthcare practitioners in choosing the most appropriate treatment for radiation-induced emesis; however, all therapies must be individualized based on the patient's needs, characteristics, and other factors.
REFERENCES
1. Feyer PC, Maranzano E, Molassiotis A, et al. Radiotherapy-induced nausea and vomiting (RINV): MASCC/ESMO guideline for antiemetics in radiotherapy; update 2009. Support Care Cancer. 2011;19(suppl 1):S5-S14.
2. Urba S. Radiation-induced nausea and vomiting. J Natl Compr Canc Netw. 2007;5(1):60-65.
3. Maranzano E, De Angelis V, Pergolizzi S. A prospective observational trial on emesis in radiotherapy: analysis of 1020 patients recruited in 45 Italian radiation oncology centres. Radiother Oncol. 2010;94(1):36-41.
4. Scarantino CW, Ornitz RD, Hoffman LG, Anderson RF Jr. On the mechanism of radiation-induced emesis: the role of serotonin. Int J Radiat Oncol Biol Phys. 1994:30(4):825-830.
5. Franzén L, Nyman J, Hagberg H, et al. A randomised placebo controlled study with ondansetron in patients undergoing fractionated radiotherapy. Ann Oncol. 1996;7(6):587-592.
6. Spitzer TR, Friedman CJ, Bushnell W, et al. Double-blind, randomized, parallel-group study on the efficacy and safety of oral granisetron and oral ondansetron in the prophylaxis of nausea and vomiting in patients receiving hyperfractionated total body irradiation. Bone Marrow Transplant. 2000;26(2):203-210.
7. National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines). Antiemesis. Version 1.2019. www.nccn.org/professionals/physician_gls/pdf/antiemesis.pdf. Accessed August 15, 2019.
8. Hesketh PJ, Kris MG, Basch E, et al. Antiemetics: American Society of Clinical Oncology Clinical Practice Guideline update. J Clin Oncol. 2017;35(28):3240-3261.
9. Ruhlmann CH, Jahn F, Jordan K, et al. 2016 updated MASCC/ESMO consensus recommendations: prevention of radiotherapy-induced nausea and vomiting. Support Care Cancer. 2017;25(1):309-316.
To comment on this article, contact rdavidson@uspharmacist.com.
Source: https://www.uspharmacist.com/article/management-of-radiationinduced-nausea-and-vomiting
0 Response to "How Long Does Nausea Etc Continue After Starting 2000iu D3"
Postar um comentário